Electrochem Seminar - "The Role of Ion-Correlation in Reducing the Lithium Transference Number in Lithium-Ion Polyelectrolyte Solutions"
Electrochem Seminar - "The Role of Ion-Correlation in Reducing the Lithium Transference Number in Lithium-Ion Polyelectrolyte Solutions"
Abstract
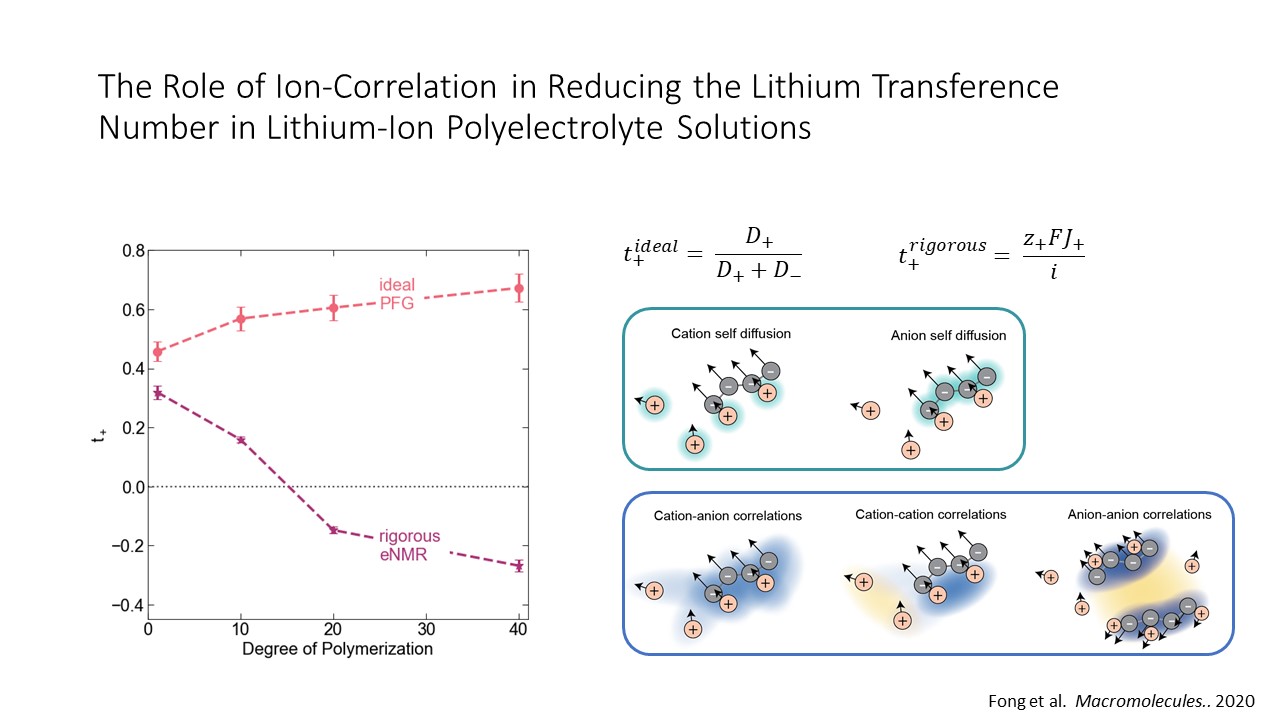
Non-aqueous polyelectrolyte solutions (PESs) have been suggested as a promising route to high conductivity, high transference number (t+) electrolytes. State-of-the-art liquid electrolytes suffer from low t+, meaning the majority of ionic conductivity results from motion of the anion rather than the electrochemically active Li+ ion. Increasing t+ requires decreasing the mobility of the anion, which is dictated by both the diffusion of the anion as well as its net charge. PESs are intuitively appealing because anchoring the anion to a polymer backbone slows down the motion of the electrochemically inactive anion while maintaining higher ion conductivity through improved ion dissociation and solvent-mediated Li+ transport. However, in polyelectrolyte systems, increasing molecular weight both decreases polymer diffusion and increases charge, which will act as competing effects for t+. Recent molecular dynamic simulations of PESs have highlighted the critical importance of correlated ion motion in these systems and have called into question oligomeric PESs as a feasible strategy to achieving high t+ and conductivity electrolytes
In this work we discuss complete studies of transport properties in lithium-ion and lithium metal battery-relevant PESs- specifically lithium triflimide appended polystyrene (PS-LiTFSI) dissolved in carbonate blends. All prior PES experimental work in the literature has relied on ideal solution assumptions for measuring transport properties. This work represents the first rigorous characterization of transport properties for a battery-relevant polyelectrolyte solution. Using electrophoretic NMR and electrochemical experiments, we characterized the transport properties, including the electrophoretic ion mobilities, conductivity, diffusion coefficients, and t+ of these model PESs. While previous studies that rely on ideal assumptions predict that PESs will have higher t+ than monomeric solutions, we demonstrate that below the entanglement limit, t+ decreases with increasing degree of polymerization. For higher degrees of polymerization, we directly observe Li+ move in the “wrong direction” in an electric field, evidence of a negative transference number due to correlated motion through ion clustering. Using calculated Onsager transport coefficients and insights from molecular dynamics modeling, we demonstrate that despite selectively slowing anion motion using polyanions, anion-anion correlation through the polymer backbone and cation-anion correlation through ion aggregates reduce the t+ in non-entangled PESs.
Speaker
Helen BergstromHelen is a 5th year Chemical Engineering PhD student in Bryan McCloskey’s group at UC Berkeley and LBNL. Helen's current research focuses on understanding and experimentally measuring transport and ion-correlation in polyelectrolytes for Li-ion batteries with a specific focus on advanced nuclear magnetic resonance spectroscopy techniques. Helen received her B.S. in Chemical & Biochemical Engineering from Brown University where she studied catalyst design for bio-oil deoxygenation. Prior to joining Bryan's lab, Helen spent 3 years as a Research Engineer at Saint-Gobain studying structure-property-processing relationships in fluoropolymers films and composites leading to two patents.